
Photo by Ben Polimer.
Introduction
Effective July 1, 2010, the state of Connecticut banned the use of all EPA-registered lawn care pesticides labeled for use on lawn (including athletic fields), garden and ornamental sites on the grounds of public and private daycares and schools grades pre-K through 8. The text of the law (C.G.S. Sec. 10-231) can be read at School IPM | Integrated Pest Management (uconn.edu) or at: Integrated Pest Management (ct.gov) (Click on “General Guidance,” then “Pertinent Statutes...”).
The only pesticides that can legally be applied in these locations are those classified as EPA 25b minimum-risk products and that do not have an EPA registration number.* Data on the efficacy of these products are still limited. Retail brands containing these EPA minimum risk ingredients have been introduced into the market, but are short-lived in availability or distribution. Therefore, use of Integrated Pest Management (IPM) and an emphasis on sound cultural practices has become even more important to school grounds managers in lieu of registered pesticides usage.
Under the current law, most pesticides that have an EPA registration number, whether synthetic or organic, cannot be used on daycare/K-8 school properties. Sanitizers, disinfectants, antimicrobial agents, pesticide bait, and aerosol sprays to protect from imminent danger from stinging or biting insects are not prohibited under this law. Products that have not been registered with the state are not allowed for use on any school grounds, including homemade remedies (e.g., the non-labeled use of vinegar, clove oil, or a “home” remedy to control a pest is illegal). Any pesticide product applied on school grounds must be listed on CT DEEP’s approved list and applied by a CT licensed pesticide applicator. Violators of CT’s pesticide law can be fined up to $5000 and/or imprisoned up to one year.
*Legislation passed in 2015 extended the pesticide ban to municipal playgrounds and permitted use of some EPA registered pesticide products to be used (Campbell and Wallace, 2020), including:
- Horticultural oils and soaps that are registered with the EPA.
- Naturally occurring substances that control pests by nontoxic mechanisms (i.e., microbial – a living microorganism is the active ingredient - or biochemical pesticides) that are registered with the EPA.
Refer to DEEP or EPA for clarification about these products.

The purpose of this document is to provide research-based guidelines for managing cool-season athletic fields most effectively, given the pesticide-free restriction. Throughout this document, current research and up-to-date information has been utilized to provide sound agronomic recommendations. This publication will address the five primary cultural practices critical for managing pesticide-free, cool-season athletic fields; 1) mowing, 2) fertilization, 3) cultivation, 4) pest control and 5) irrigation. In addition, turfgrass selection, aggressive overseeding, and compost topdressing will be discussed.
Pesticide-free management fundamentally changes pest control. Until July 1, 2010, sports turf managers incorporated various chemical controls into their maintenance programs to manage weeds, insects, and diseases preventatively and/or curatively. Since most chemical controls are no longer an option for school grounds managers, there has been tremendous confusion regarding effective methods to control invasive weeds, damaging insects and potentially devastating diseases. This is a concern for all turfgrass managers that maintain high quality turfgrass, but particularly for sports turf managers. Athletic fields present a different challenge compared to other highly maintained turfgrass areas, due the nature of the traffic they endure and the liability associated with their use. Athletic fields are in a constant state of re-establishment. Intense traffic and the subsequent reduction in turfgrass cover create an environment that is optimal for pest encroachment. Reduced turfgrass cover allows weed seeds to germinate and remain competitive. Loss of vegetative cover has also been associated with increased playing surface hardness and risk of injuries (Dest and Ebdon, 2011). Player safety and field aesthetics can be significantly compromised when dominated by weeds, such as large crabgrass (Digitaria sanguinalis L.) and white clover (Trifolium repens L.) (Brosnan et al., 2014). Turfgrass disease and/or large populations of damaging insects may turn a well-established turfgrass stand into an unstable playing surface very quickly.
Implementing sound cultural practices throughout the year is critical to maintain healthy turfgrass so the playing surface will be uniform, safe and in the best possible condition when used by teams or student classes. Healthy turfgrass will be more tolerant of stress related to intense traffic or drought, as well as weeds, insects, and diseases.
Assessing Playing Surfaces
While all natural grass fields require attention directed toward the five cultural practices mentioned above, the level of maintenance can be adjusted based on usage and available resources. To understand the need for maintenance, each field should be assessed for safety and overall health. The UConn Field Assessment Form enables turfgrass managers to assign a quantitative value to overall field health and playing surface conditions.
Prioritizing Playing Surfaces
Prioritize athletic fields for maintenance based on level of care, amount of use, and desired turfgrass quality. There are three categories: High, Medium, and Low (Park, 2014; Park and Murphy, 2014).
- High Priority Fields (Varsity or “Game Day Ready” fields and practice fields): high maintenance inputs and irrigation required with immediate repair needs. These fields need to provide the highest level of turfgrass health, safety and aesthetics.
- Medium Priority Fields (Varsity/Junior Varsity fields): medium maintenance inputs, immediate repair needs. These can be practice or multi-use fields in high visibility locations. Safe playing surfaces are still mandatory. Irrigation preferred, but might not have an automated irrigation system.
- Low Priority Fields (Recreational Multi-use fields/General Turf Areas): These fields or lawns can be managed with minimal maintenance and no irrigation.
Mowing
Ideal mowing height depends on multiple factors, such as sport, turfgrass species, irrigation, and field use (Table 1).
| Table 1. Factors affecting mowing height selection (Adapted from ASTM, 2005). | ||
| Athletic Field Use | Grass Species | Mowing Height* |
| Baseball, Softball, Soccer, Football | Kentucky bluegrass (KB), perennial ryegrass (PR), or KB/PR mixture | 1.5-2.5 in. |
| Intramural and Multiple use fields | Kentucky bluegrass, perennial ryegrass, KB/PR mixture, or tall fescue | 2.0-3.5 in. |
| *If the field is not irrigated, the turfgrass should be mowed at the high end of the suggested range. | ||
| Mowing heights can be increased (0.5-1.5 in.) during the off-season and/or summer months to decrease the mowing frequency and help reduce weed encroachment. However, reducing the mowing height to competition height should begin 4-6 weeks before the first game/practice (Puhalla et al., 1999). Mowing height of cut (HOC) should be reduced gradually (Vanini and Rogers, 2008) (e.g., 0.5 in. at a time, allowing 3-5 days between mowing). Mow each HOC at least twice before reducing the HOC further. | ||
For scheduled maintenance purposes:
- Mow consistently at least twice per week (Calhoun et al., 2002), removing no more than 1/3 of the leaf blade. Maintaining a regular mowing schedule is critical, especially when cool-season turfgrasses experience the seasonal flush of growth that occurs both in spring and fall. During this seasonal time of rapid growth, or depending on priority use of the field, mowing 3x per week may be necessary.
- The sport may dictate the frequency of mowing schedule and type of mower used.
- A higher mowing height can help reduce weed incidence (Calhoun et al., 2005). However, athletic fields constantly subjected to traffic create voids in the turfgrass canopy, allowing opportunistic weed to germinate. Weed pressure will be constant, particularly in areas of high traffic.
- For non-irrigated fields, mowing at the highest height of cut recommended for cool-season turfgrass species, such as Kentucky bluegrass (KB) or perennial ryegrass (PR), is critical. A higher height of cut will allow for a better root system, which will support the turfgrass as soil conditions dry. If the height of cut is too low, the root system will be compromised. Shorter root systems challenge turfgrasses and make them more susceptible to stresses that can diminish available turf cover.
- Returning clippings can reduce fertilizer requirements, particularly nitrogen (up to 50%), without decreasing turfgrass quality in mixed turfgrass stands containing predominantly Kentucky bluegrass and perennial ryegrass (Kopp and Guillard, 2002).

- Alternate the mowing pattern with each mowing event to reduce potential wear patterns that will provide areas for weeds to germinate and grow.
- Mow when turfgrass is dry to maximize clipping dispersion. Regularly sharpen and adjust mower blades or reels to cleanly cut turfgrass leaf blades rather than rip or tear leaf blades. Mowers used each day will require sharpening at least once a week. Mowers consistently used on sandy fields may also require an increase in the frequency of blade sharpening.
- Remove excess clippings from mower blades before exiting a field.
- Some approved 25(b) control products may impact pollinator health (e.g., Beauveria bassiana strain GHA (e.g., BotaniGard ES, Mycotrol WPO). Mowing before any application is made in athletic fields to remove flowers would deter bees from foraging in the area and help to prevent pollinators from ingesting chemicals as they forage. As with synthetic products, check the label of any minimum risk product to ensure that the product is used in a way that protects bee health.
Fertilization
Nitrogen (N) is the element that has the greatest impact on turfgrass growth and vigor. Age of the field, health of the turfgrass stand, level of field usage, availability of irrigation, clipping management, and soil organic matter all must be considered when developing a nutrient program for an athletic field.
According to Connecticut law, no phosphorus (P) fertilizer applications can be made Dec. 1-March 15. Phosphorus applications are not permitted unless a soil test within 2 years indicates a need for P. Applications of P are permitted without a soil test during establishment or if the fertilizer source contains <0.67% phosphate (P2O5).
Apply N at a rate of 3-4 lbs N/1000 ft2/year and fertilize frequently when using soluble sources (6-8 applications, April-October) on 21-28 day intervals (Calhoun et al., 2002). Apply no more than 0.5 pound of soluble N/1000 ft2 per application only when weather conditions permit. Apply when leaf tissue is dry and then irrigate immediately after application. Slow-release N sources (such as poly-coated sources) can be applied at higher rates less often (e.g., 1.5-2.0 pounds N in mid-May and 1.5-2.0 pounds N in Aug.). Organic fertilizers are also an option, however, they often contain P and may contain a smaller amount of N compared to synthetic fertilizers. If no P is recommended as part of the soil test recommendations, no P should be applied.
Soil test every 1-3 years to monitor soil nutrient levels and pH. Follow soil test recommendations for lime, P, and potassium (K). Maintain P levels at the low end of the “optimal” range to discourage annual bluegrass encroachment. The optimum range for P is 6-20 lbs per acre (modified Morgan). Consistency in results is key to reduce confusion when following nutrient recommendations. It is advisable to use one reliable source when receiving corrective recommendations; in-state university soil laboratories are recommended. Results from other laboratories may vary due to the method used to measure soil available P.

Maintain soil pH at 5.9-6.5 to maximize nutrient availability. In Kentucky bluegrass swards with pH greater than 6.5, consider applications of ammonium sulfate or sulfur coated urea from late May to July to help reduce pH levels in the root zone and minimize summer patch severity.
Fertilizer spreader calibration and application data can be easily completed and captured using the UConn FertAdvisor smart phone app. The app was recently updated to store historical data for record keeping and future retrieval. The app is available for both Android and Apple platforms.
Tips for Obtaining a Representative Soil Sample (Instructional video in FertAdvisor App):
- Best time to sample is late fall or prior to the next fertilizer application.
- Select 20-25 random locations across the field.
- Sample to a consistent 4 in. depth using a soil probe or small shovel.
- Remove and discard thatch and turfgrass from each subsample.
- Mix subsamples in a clean plastic bucket.
- Transfer one cup of soil mixture into a secure bag for shipping.
UConn Soil Testing Lab: Home | Soil Nutrient Analysis Laboratory (uconn.edu)
Cultivation
Cultivation practices may be part of the routine maintenance to reduce compaction of intensively used athletic fields. Regular cultivation is necessary to reduce soil compaction, increase infiltration rates, increase gas exchange, reduce organic matter accumulation, and maintain acceptable levels of surface hardness. Frequency of cultivation is based on field compaction and use. If warranted and recommended, cultivation can be targeted to specific areas within a field, rather than the entire field. The cultivation process can be stressful to the turfgrass plant and is recommended when the plant has the opportunity to grow and recover.
The following dates and cultivation types are recommended for athletic fields predominately used in the fall. Deep tine cultivation (any type of cultivation that reaches depths of greater than 6”), should be completed every 3 years to break up any subsurface compaction in the soil profile.
Hollow-tine Cultivation (April 15-May 15)
HTC helps to alleviate soil compaction at the surface, increase infiltration rates, and prepare a quality seedbed for spring re-establishment efforts. The window for HTC is recommended to maximize turfgrass and root recovery following cultivation. Additionally, HTC has also been shown to provide some mechanical control for white grubs (McGraw and Holdrege, 2012). The opportunity to inflict damage to the grubs in the spring exists when white grubs are actively feeding (located in the 3-4” soil depth range), generally from April 15 through June 1. Further delaying cultivation decreases root growth recovery time and extends the active feeding period.

Solid-tine Cultivation (August 1-Sept 15)
STC is preferred at this time of year, causing less surface disruption than HTC with anticipated heavy fall field usage. STC creates some voids at the surface for gas exchange and water movement, and will also provide some mechanical control for white grubs (McGraw and Holdrege, 2012). Generally, in the fall, white grubs are actively feeding (located in the 3-4” soil depth range) from August 1 through November. Therefore, as mentioned above, the opportunity to inflict damage to the grubs best occurs during this time. Further delaying cultivation extends the active feeding period and negatively impacts turfgrass recovery as growth slows with reduced temperatures.
Athletic fields primarily used in the spring require a reverse cultivation strategy (solid-tine cultivation (STC) in the spring and hollow-tine cultivation (HTC) in the fall).


Tips to Optimize HTC and STC Efficacy:
- Use 5/8 in. tines, 2 in. × 2 in. spacing, 2 passes.
- Use only a PTO-powered, vertically operating unit to provide consistent tine penetration depth to 3-4 in.
- Return cores using a heavy drag mat (HTC only).
Pest Control
Site specific pest management treatments are recommended to maintain the integrity and safety of the playing surface and to reduce the risk of injury to field users.
Weeds
Weeds were identified as the number one pest concern in a 2010 survey of school grounds managers (Bartholomew et al., 2016). The best overall defense against weed encroachment is maintaining a healthy turfgrass stand through proper mowing and fertilization. Applying nitrogen alone (3 lbs N/1000 ft2 per year, compared to none) has been shown to significantly reduce white clover and dandelion infestations (Calhoun et al., 2005).
Weed infestation can negatively affect playing surface characteristics. Aggressive overseeding has been shown to significantly reduce weed populations (grassy annuals and perennial broadleaves) in pesticide-free management programs (Elford et al., 2008; Miller and Henderson, 2012). Overseeding is currently the most effective pesticide-free method for reducing weed populations (see the aggressive overseeding section).

At the present time, there are no documented, selective, post-emergent, EPA minimum risk 25(b) products to control weeds available to K-8 school grounds managers. Fiesta, an organic, EPA registered (and therefore not allowed for use on school grounds in CT), chelated iron product has demonstrated moderate control of white clover after repeated applications and also will darken turfgrass (Smith-Fiola and Gill, 2014). The only products currently available for use are nonselective, contact defoliants of leaf and stem tissue. Re-growth of most weeds (and turfgrass) naturally occur within 3-4 weeks; therefore, multiple, repeated applications are required throughout the season. Ingredients in some approved 25(b) minimum risk products (clove oil, citric acid, and/or acetic acid) are considered to have some post-emergent weed control activity. These products will damage the surrounding turfgrass, so care must be taken when spot treating weeds on actively used athletic fields. It may be practical to combine a spot treatment with an overseeding application to fill in voids created whennthis material is applied. Burndown of these products was found to be most effective when applied in full sun and high temperature (Neal and Senesac, 2018). Repeated application of these burndown products may be used in areas such as fencelines, warning tracks, infields, batting cages, under stadium seats, and team dugout areas where damage to turfgrass surfaces is not a concern.
Corn gluten meal (CGM), an organic, pre-emergent herbicide that exhibits root-inhibiting properties and also contains nitrogen (usually 9-10%), has been shown to significantly reduce crabgrass germination when applied at the recommended rate (20-25 lbs/1000 ft2) (Christians, 1993). However, crabgrass suppression was not effective in other research (Miller and Henderson, 2012; St. John and DeMuro, 2013). CGM has herbicidal activity, preventing root formation of some germinating plants. Therefore, efficacy can be enhanced when CGM-affected weeds are subjected to a period of water stress.
Tips to Optimize CGM Efficacy (Christians, 2002)
- Apply in the spring 2-4 weeks before summer annuals (e.g., crabgrass) germinate. Crabgrass will germinate when the soil temperature reaches 55° F. Once soil temperatures consistently reach low to mid 50’s, apply CGM at the recommended rate (20 lbs/1000ft2). This results in approximately 2 lbs N/1000 ft2 per application.
- If no precipitation occurs within 5 days of application, apply 0.25 in. water.
- Following weed seed germination, do not irrigate to encourage weed desiccation. Weeds will germinate, forming only a shoot, but not a root. If the soil is too wet, the weed can recover and form a root.
- CGM will inhibit turfgrass germination when applied at seeding. Inhibitory effects will last for approximately 5-6 weeks. Therefore, CGM should not be used within 6 weeks before desirable grasses are seeded. If overseeding is required in the spring, timing of CGM application is critical. CGM should not be applied until all turfgrasses germinate.
- The N will release slowly over a 3-4 month period following application. A follow-up application can be made in August to help control some perennial weeds germinating in late summer, while providing an additional 2 lbs N/1000 ft2
An alternative weed control strategy in high wear areas is reestablishment using sod. Weed establishment was consistently lower with sod as a treatment cover, compared to hydro-mulching, hydro-seeding, or dry seeding applications (Jordan and Lyons, 2010). In fact, used as an alternative strategy to manage for broadleaf weeds in residential lawns, weed populations managed through the use of sod consistently had less than 5% of weed populations for multiple years, compared to other management treatments in controlled studies (Siva, 2014). Sod establishment in early December can provide adequate root development for subsequent spring field usage, providing an alternative field renovation opportunity when field use is typically low (Henderson et al., 2009).
Insects
Frequent scouting for insect presence should be completed as part of a comprehensive management plan. This will enable turfgrass managers to alter their cultural practices (e.g., increase frequency of irrigation cycles and fertility applications) to help mask the damage and encourage turfgrass recovery.
Surface feeding
Surface feeding insects that present the greatest potential for damage in cool-season turfgrasses are chinch bugs (Blissus leucopterus hirtus), sod webworms (Herpetogramma spp.), and bluegrass billbugs (Sphenophorus parvulus). An effective strategy for deterring these pests is selecting turfgrass seed mixtures or blends that contain endophyte-enhanced, improved cultivars of perennial ryegrass and/or tall fescue. Additionally, many surface feeding insects thrive in thatch. Manage thatch depth (<0.5 in.) utilizing core cultivation, verticutting, and topdressing (see cultivation section).
With the 2015 amendment to the pesticide law, the use of microbial products on school properties is now permitted. A fungal bioinsecticide, Beauvaria bassiana strain GHA (trade names-BotaniGard ES, Mycotrol WPO) is now an available pest control product that targets many surface feeding insects of both turf and ornamentals, including chinch bugs and billbugs. However, this product is also toxic to bees; therefore, it should not be used where pollinators are present. It is recommended that an athletic field be mowed prior to the application to remove weed flowers and discourage bee foraging. Chromobacterium subtsugae (trade name-Grandevo PTO), is another bacterial bioinsecticide product, also reported to reduce populations of chinch bug and sod webworm. As with any pesticide product, it is important to read the label for application directions. For more information and options, refer to Biological Pest Control Products Available for Connecticut School Grounds (ipm.uconn.edu).
Root feeding
White grubs are considered the most destructive insect pest of cool-season turfgrasses. For high priority pesticide-free athletic fields, manage to ensure optimum turfgrass health and be vigilant with in-season scouting practices, if grub activity is suspected. Endophyte-enhanced turfgrasses do not deter these root feeding insects. The white grub species most damaging in New England are European chafer (Amphimallon majale), Oriental beetle (Anomala orientalis), Japanese beetle (Popillia japonica) and Asiatic garden beetle (Maladera castanea).
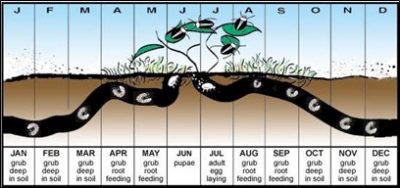
Life cycle
These scarab beetle species have an annual (1-year) life cycle. While there is variation in the timing of the adult emergence of these beetle species, in general, adults emerge late May to late June, mate and lay their eggs from late June to mid-late August. Eggs hatch in 2-3 weeks and larvae begin feeding immediately and will continue to feed until the first frost. Visible turfgrass damage occurs once larvae have grown to second or third instars (Sept-Oct), and where there are >15 larvae per ft2. Acceptable threshold levels are likely much less for athletic fields, due to surface stability concerns. This warrants early scouting (Aug. 1-Sept.1) prior to signs of turfgrass damage. Once soil temperatures drop in the fall, the grubs move deeper into the soil to overwinter. Grubs will resurface in early spring (March or April) to resume feeding until they pupate in late May/early June (Potter, 1998).
The life cycle of each white grub species differs enough that the correct identification of the grub is essential to appropriately time an application at the most vulnerable stage of the insect’s life cycle. This will result in the most effective control. Entomopathogenic nematodes (EPN), Heterorhabditis bacteriophora (trade names-Nemashield HB, Nemasys, Nemaseek) and Steinernema scarabaei (trade name-Nemagard), are microscopic, worm-like organisms that can be used to control white grubs. EPNs enter the white grub’s body and then reproduce with the help of a symbiotic bacterium. This results in the death of the infected white grub and additional cycles of infection in other grubs. EPNs live in the upper 10 in. of soil and are often applied as a liquid suspension directly onto the playing surface. EPNs are often applied curatively, however, in areas where white grubs have previously been a problem. Consider preventative EPN applications in late July/early August. These nematodes target immature grubs which are most vulnerable to infection. When using EPNs as a treatment, care must be taken to maximize nematode survival until they reach the grub host.
Tips to optimize infection rate of grub host:
- Select the best timing of application - Late July through mid-September provides an immature host and ideal soil temperatures for infection (nematodes are more active and bacteria replicate more readily under soil temperatures above 65 degrees F) (Grewal et al., 2004).
- Purchase nematodes as close to application date as possible due to their short shelf life.
- Irrigate area immediately before, during treatment, and following an application of nematodes.
- Pre-soak the area prior to EPN application if the soil is dry. Adequate soil moisture provides optimum conditions for nematode survival because EPNs travel in a film of water on soil particles to reach their hosts. Maintaining adequate soil moisture 2-3 days following an application can enhance efficacy. Do not saturate the soil, as low oxygen conditions can be lethal for the nematodes.
- Do not use sprayers with piston pumps, as these can injure nematodes.
- Remove screens from spray tips to prevent clogging.
- Do not allow spray water containing nematodes to overheat.
- EPNs are very sensitive to UV light. Spray in the evening or on cloudy days.
With the change in the 2015 law, bacterial bioinsecticide products are now permitted for use on school grounds. Bacillus thuringiensis (Bt) var. galleriae, is a naturally occurring soil bacterium that targets and kills white grubs. Sold as “grubGONE,” the bacterium attacks the larval beetle stage and can be used on athletic fields if populations of young grubs are detected (“beetleGONE” is appropriate for treating adult beetles in a landscape setting).
Diseases
A turfgrass disease outbreak requires a susceptible host, virulent pathogen, and a conducive environment. Although pesticides are not an option, the turfgrass manager can minimize disease incidence through proper turfgrass selection and cultural practices to make the environment less favorable or disease.
Turfgrass Selection
In a pesticide-free environment, options to control turfgrass diseases can be limited. Turfgrass managers can minimize disease incidence through proper turfgrass selection. Utilize turfgrass mixtures (seeding more than one species, e.g., Kentucky bluegrass/perennial ryegrass) and/or blends (using more than one cultivar of the same species) to increase the genetic diversity of the turfgrass stand. Select disease resistant cultivars to help prevent the establishment of certain diseases. The National Turfgrass Evaluation Program website (NTEP.org) provides disease resistant ratings of current turfgrass cultivars. Selecting seed mixtures or overseeding turfgrass cultivars that have been developed for resistance to specific disease is critical, particularly if confirmation of the disease has been recorded. Recent evaluations of turfgrass seed mixtures at Rutgers, has demonstrated that mixtures of different turfgrass species or specific cultivars can substantially reduce the incidence of turfgrass diseases, such as summer patch or brown patch (Park et al., 2017).
Although conventional pesticides are not an option, biological fungicides are now permitted to be used on school properties. The UConn Turfgrass Pathology lab (cahnr.uconn.edu/turflab) is available to confirm turfgrass disease pests, and recommend alterations in management programs that will reduce disease severity. The lab is a valuable in-state resource for all turfgrass professionals. Confirmation of all turfgrass pests is necessary to ensure that the correct control product is utilized.

Environment
Leaf wetness is the most important factor when trying to limit foliar diseases (Smiley et al., 1993). Irrigation should be applied early in the mornings (i.e., 3-6 am) when the canopy is likely already wet with dew. Avoid irrigating during early evening before dew forms, or late in the morning. Irrigating at these times will likely extend leaf wetness duration and encourage disease. If multiple irrigation cycles are required during the day (i.e., at establishment), the last application should allow time for leaves to dry before nightfall. Correct surface drainage (athletic fields constructed using native soil should be sloped > 1.5% and lengths of these slopes should not exceed 200 ft) and subsurface drainage problems. Water accumulation in low areas can encourage foliar and root diseases, as well as abiotic decline, particularly where thatch is excessive. Caution should be exercised when managing diseased areas to prevent further dissemination of pathogens. Equipment should be washed when moving from infected fields to disease free areas to prevent further spread of disease.
Irrigation
Irrigation is absolutely essential for maintaining acceptable playing surface quality on natural turfgrass athletic fields. The lack of irrigation can dramatically impact management strategies that support turfgrass recovery, playability of the field, and player safety. In the absence of irrigation, the expectations for playing surface quality should be dramatically reduced. Due to intense traffic, athletic fields are in a perennial state of re-establishment. Adequate moisture is necessary to initiate and complete the germination process, and encourage seedling development. Irrigation is also imperative for the success and ease of completing other cultural practices, such as fertilization, cultivation, and pest control. Light and frequent irrigation can also be extremely helpful during recovery from insect damage, disease damage, or intense traffic.
Utilize wilt-based irrigation, or wait until mild drought stress is visible (leaf folding and foot printing), before irrigating to replace moisture lost by evapotranspiration (ET) for maintaining established, mature, healthy turfgrass stands (Lewis et al., 2012).

If an ET based in-ground irrigation system is not available, self-retracting water reels are an easy to use, inexpensive substitute. However, water reels need to be closely monitored while using to ensure proper operation. Regardless of system type, ensure all irrigation heads are operating correctly and coverage is uniform. Fields that are irrigated should be regularly audited to ensure water is being applied appropriately to all portions of the playing field.
Aggressive Overseeding
Aggressive overseeding is one of the most important cultural practices required to maintain acceptable turfgrass quality and playing conditions on high traffic/pesticide-free athletic fields. Aggressive overseeding can be defined as applying seed (at rates exceeding the typical recommended ranges for seedling establishment) onto well-established turfgrass areas, regardless of turfgrass density, in an effort to maintain/increase desirable species on athletic fields subject to intense traffic (Minner et al., 2008). Intense traffic and evidence of wear at key locations of the field is typical of seasonal sports. Preemptively overseeding areas of the athletic field that become thin or worn before permanent turf cover is lost is critical in maintaining safe playing surfaces. Selection of turfgrass species and the timing of the overseeding application depends on the athletic field’s intensity of use (Table 2). Given budgetary restrictions, overseeding efforts can be focused on concentrated traffic areas. Perennial ryegrass (PR) is the preferred turfgrass species for aggressive overseeding, due to its quick germination, speed of establishment, and ability to develop under heavily trafficked conditions (Minner et al., 2008). Recent overseeding research of non-irrigated, pesticide-free athletic fields in CT also confirmed perennial ryegrass as the preferred species for in-season repair and overseeding on heavily-used, non-irrigated, pesticide-free athletic fields (Maxey et al., 2020). Renovated or overseeded fields that are allowed to establish without activity are afforded more opportunity to incorporate other species. There has been a growing consideration to include Tetraploid ryegrass into perennial ryegrass blends for overseeding in late fall. Tetraploid ryegrasses demonstrate success at germinating at cooler soil temperatures (< 50o F) compared to >50o F temperatures required for perennial ryegrass. Characteristics such as good winter hardiness and root growth in cool soils could potentially help repair worn fields damaged in the late fall and aid in early spring establishment before other cool-season turfgrasses resume growth.
| Table 2. Overseeding strategies based on time of athletic field use. | ||
| Season of Predominant Athletic Field Use | Overseeding Strategy | |
| April 25 – May 10 | Sept 1 – Sept 15 | |
| Fall | 70-30 (KB:PR)* | 100% perennial ryegrass† |
| Spring | 100% perennial ryegrass | 70-30 (KB:PR) |
| * Target seeding rate for the 70-30 KB/PR mixture is 3lbs/1000ft2 | ||
| †Target seeding rate for the 100% PR is 20lbs/1000ft2 | ||
A KB/PR (70-30) mixture (by weight) for spring re-establishment is recommended to help maintain KB populations on a fall use athletic field (Stier et al., 2008). Conversely, seeding KB/PR in the fall for spring use of an athletic field is also a viable option. Intense wear in the fall, which may discourage the re-establishment of KB by overseeding, may require that KB be introduced as sod and overseeded with PR.
If tall fescue (TF) is desired as the primary turfgrass species, the field should not be used for at least 14 weeks to allow the turfgrass to establish (Shelley and Serensits, 2012). If there is not an appropriate time for establishment when the field is out of use, a sod base of TF can be introduced late in the season after all field activity has ceased. Maintaining multiple species on the playing surface (e.g., KB/PR or TF/KB) helps maintain genetic diversity, which is important for disease management and maintaining turf density.
Selecting the most appropriate turfgrass species and cultivars is critical to enhancing the quality, performance and longevity of the playing surface. Evaluation of turfgrass species and cultivars has long been coordinated by the National Turfgrass Evaluation Program (NTEP.org). Many testing locations now evaluate wear tolerance as a component of cultivar performance. Within the last 10 years, turfgrass seed companies have actively engaged university cooperators to evaluate turfgrasses based on specific environmental performance. The Turfgrass Water Conservation Alliance (tgwca.org) has a focused interest towards turfgrass performance and reduced water needs. The Alliance for Low Input Sustainable Turf (a-listturf.org) evaluates turfgrasses that perform well with reduced cultural (water, fertility, and chemical) inputs. These evaluation trials should be consulted when developing seed mixtures for athletic field use, particularly for pesticide-free fields.

For in-season maintenance, seed should be broadcast immediately prior to a cleated practice or game, allowing players to work seed into the soil and optimize seed-to-soil contact. If traffic is not imminent, seed should be applied using a spike seeder, which will maximize seed-to-soil contact while minimizing damage to the existing turfgrass stand. If the athletic field is used May-August, apply 3-5 lbs of PR seed/1000ft2 per month.
In late August or September, apply 20 lbs seed/1000ft2. Research has shown that applying seed as a single, early application of PR more than doubled the amount of turfgrass cover, compared to dividing the same amount of seed into multiple, smaller amounts applied each week before a game or practice (Minner et al., 2008). The total target rate is 35-45 lbs of seed/1000ft2 per growing season (turfgrass species selection depends on field use, time of year, and presence of irrigation).
For PR, select 3-4 cultivars to create a seed blend. Select 1-2 cultivars that have medium to high wear tolerance and 1-2 cultivars that have grey leaf spot resistance. Consider other cultivar qualities, such as spring green-up, cold tolerance, and drought tolerance. Please refer to ntep.org for cultivars that have been evaluated in your region. University Extension Specialists are a valuable resource for more information.


Compost Topdressing
Topdressing with compost as part of an athletic field management program requires careful consideration. Routine topdressing with compost is not recommended unless the soil is monitored for soil test phosphorus values by testing the soil 1-3 months after every topdressing application. Contrary to many current recommendations, compost topdressing should not be considered an essential component to a pesticide-free management program. However, compost can have beneficial effects. Research has shown topdressing with compost can: 1) help retain greater percent cover after wear, 2) decrease bulk density, 3) increase water retention, and 4) decrease surface hardness (McNitt et al., 2004; Tencza and Henderson, 2012). However, compost topdressing can be labor intensive to apply and it is easy to apply excessive amounts of P when manure based composts are applied. Additionally, when applying compost as topdressing, incorporation into the soil is extremely difficult.
Consider the following:
- Soil test to determine current soil phosphorus levels. Modified Morgan extractable P values should be less than 20 lbs/acre.
- Fields with values greater than 20 lbs/acre should not receive P applications from fertilizer compost or soil amendments.
Fields constructed with native soils will have greater moisture holding capacity if soil organic matter (SOM) content is 4-6%. If the SOM is <4%, and the soil test P value is less than 20 lbs/acre modified Morgan extractable P, consider compost applications to increase SOM levels into the 4-6% range. Use a compost low in P (<0.3% P2O5), and test soil for P 1-3 months after every compost application (pull 1-inch soil samples). Amounts of P applied for various depths of application and concentrations of P in compost are listed below (Table 3). If the field is a sand-based constructed field, increasing SOM content greater than about 1% is not recommended.

Tips for Responsible Compost Applications:
- Soil test to determine current soil P levels. Modified Morgan extractable P values should be less than 20 lbs/acre.
- Use only compost sources containing less than 0.3% phosphate (P2O5), such as leaf compost.
- Test compost before purchase for organic matter quality (ASTM, 2002). Target ranges for desirable compost characteristics are listed below (Table 4).
- Screen compost before application to at least 1⁄2 in. (1⁄4 in. preferred)
- Topdressing should coincide with hollow-tine cultivation to help mix compost with the existing soil (See cultivation section).
- Apply no more than 1⁄4 inch of compost per topdressing.
- Test soil for P 1-3 months after every compost application (pull 1-inch soil samples).
| Table 3. Total pounds of phosphate (P2O5) applied per 1000 ft2 using composts with various percentages of P concentration when applied at various rates. | ||||||||
| Application rate | Percent Phosphate (P2O5) in Compost | |||||||
| Depth (in.) | Yd/Acre* | Tons/Acre | 0.05% | 0.5% | 1% | 1.5% | 2% | 2.5% |
| ------ Pounds phosphate (P2O5) applied per 1000 ft2 ------ | ||||||||
| 1/8 | 16.9 | 6.8 | 0.2 | 1.6 | 3.1 | 4.7 | 6.2 | 7.8 |
| 1/4 | 33.8 | 13.5 | 0.3 | 3.1 | 6.2 | 9.3 | 12.4 | 15.5 |
| 1/2 | 67.5 | 27.0 | 0.6 | 6.2 | 12.4 | 18.6 | 24.8 | 31.0 |
| 1 | 135.0 | 54.0 | 1.2 | 12.4 | 24.8 | 37.2 | 49.6 | 62.0 |
| 2 | 270.0 | 108.0 | 2.5 | 24.8 | 49.6 | 74.4 | 99.2 | 124.0 |
| Adapted from the Composting Council, University of Missouri Extension, 2011. | ||||||||
| *Based on an average compost weight of 800 lb./cubic yard (wet weight) | ||||||||
| Table 4. Target ranges for chemical characteristics, physical characteristics and maturity level of desirable compost topdressing materials (Landschoot, 2013). | |||||||||
| Chemical Characteristics | Physical Characteristics | Maturity | |||||||
| Parameter | pH | Soluble Salts (mmhos cm-1) |
N (%) | P (%) | K (%) | C:N Ratio | Organic matter (%) | Moisture Content (%) | Respirometry (mg CO2-C/g*) |
| Target Range | 6.0-8.0 | < 6 | 0.5-3.0 | 0.2-0.9 | 0.2-0.5 | < 30:1 | > 30 | 30-50 | < 8 † |
| * mg CO2-C/g organic matter/day – Respirometry (CO2 evolution) provides a measurement of the relative microbial activity in a compost. Therefore, this can be used as an estimate of compost stability. | |||||||||
| † Interpretive index from the U.S. Compost Council Test Methods, <2 = Very Stable – Well-cured compost, no continued decomposition, no odors, 2-8 = Stable – cured compost, odor production not likely, minimal impact on soil carbon and nitrogen dynamics. | |||||||||
Other Potential Tools and Considerations
Tools
Invest in tools that support turfgrass health, decision-making protocol, and/or communication. Examples include the following:
- Portable volumetric soil moisture meters help identify the need for irrigation and/or improve water movement.
- Clegg hammer measures surface hardness and can be used to support decisions to close a field that may be a hazard for player safety.
- Utilize the UConn Field Assessment Form (ipm.cahnr.uconn.edu) or the Playing Conditions Index (PCI) from Sports Turf Managers Association (STMA) (stma.org), to document maintenance and field safety conditions. This also provides information that supports open communication with field users and administration personnel. Each school/town IPM plan also should be updated annually, posted, and available to town residents.
Growing Degree Days
The ability to scout, detect and prevent pest problems is important in the management of pesticide-free athletic fields and grounds. Tracking growing degree days (GDD) can be a particularly useful tool to support scouting efforts. Use of GDD can support cultural management strategies related to timing of weed germination and seedling emergence, disease onset, and awareness of insect emergence or development. GDD represents the daily accumulation of “heat units” based on air temperature and are summarized daily with weather stations over the growing season.
Wetting Agents
Use of wetting agents as a water management tool may help keep athletic fields playable and safe. Wetting agents aide in water infiltration, sustain turfgrass health, and improve surface firmness. Wetting agents are surfactants (soaps) that loosen surface tension of water molecules to allow for improved water movement into both native and sand-based fields. Products can be applied for season-long effect or applied for short term use. Research at Iowa State observed differences in turfgrass cover, surface hardness, and traffic events with an application of wetting agents (Pease, et al., 2018). Wetting agents also can be used in northern climates late in the season to reduce moisture related winter injury (Bauer, 2017).
Fraze Mowing
Fraze mowing is a well-documented renovation practice that can reinvigorate the establishment of turfgrass surfaces. The practice has also been observed to drastically reduce populations of weeds at the soil surface (Sitko and Rossi, 2019). This aggressive cultural practice removes a thin layer of soil and organic matter from the playing surface. A fraze mowing event, in coordination with aggressive overseeding and consistent irrigation, can produce a uniform and dense playing surface within 4-8 weeks. The field needs to be removed from activity during the grow-in/renovation and can only be considered if alternate field space is available.
Turfgrass Covers
Growth covers can enhance turfgrass germination and growth when soil temperatures prohibit rapid growth and recovery. Sunlight penetrates the cover and heats the air between the cover and the soil surface, allowing the soil to remain warm enough to encourage turfgrass growth. Moisture is also more available for the germinating turfgrass plants (Kurcab, 2018). Using turfgrass covers, turfgrass areas that are prone to heavy wear, particularly in late fall, and which are overseeded after the end of the fall sports season, can be protected and removed from play. Turfgrass covers also aid in spring establishment once soil temperatures warm and growth resumes.
Communication
Communication with administrators, athletic directors and municipal residents that wish to use town/schools fields for recreational or sporting events need to understand the level of player safety required on all school or municipal fields and how that translates to management protocol. Keeping decision makers and those that use school fields informed of maintenance practices that reduce the risk of injury and support field health is critical.
- Develop a Field Use schedule and a map of the fields.
- Post notice of when and why the fields are open or closed (e.g., general maintenance and repair, field rotation, safety, etc.).
- Place this schedule online or post in a prominent location at the school or in town.
- Designate an area/field for general use to avoid unnecessary use of designated priority fields.
- Areas for use should be rotated with other fields to manage level of wear.
Literature Cited
American Society for Testing and Materials. (2002). Standard Test Methods for Organic Matter Content of Putting Green and Sports Turf Root Zone Mixes. Annual Book of ASTM Standards. Designation: F 1647-02a. Vol. 15.07 p. 323-324.
American Society for Testing and Materials. 2005. Standard Guide for Maintaining Cool-Season Turfgrasses on Athletic Fields. Annual Book of ASTM Standards. Designation: F 2060-00 (Reapproved 2005). Vol. 15.07 p. 544-547.
Bartholomew, C., Campbell, B, & Wallace, V. (2015). Factors Affecting School Grounds and Athletic Field Quality after Pesticide Bans: The Case of Connecticut. HORTSCIENCE 50(1), 99–103.
Bauer, S. (2017). Managing Sports Turf Using Wetting Agents: a case for full field applications. sportsfieldmanagementonline.com/2017/12/19/managing-sports-turf-using-wetting-agents-a-case-for-full-field-applications/9139/
Brosnan, J. T., Dickinson, K., Sorochan, J., Thoms, A., & Stier, J. C. (2014). Large Crabgrass, White Clover, and Hybrid Bermudagrass Athletic Field Playing Quality in Response to Simulated Traffic. Crop Science 54(4), 1838-1843. doi.org/10.2135/cropsci2013.11.0754
Calhoun, R., Sorochan, L., Sorochan, J. & Rogers, J. (2002). Optimizing Cultural Practices to Improve Athletic Field Performance, Revised. Michigan State University Extension E18TURF.
Calhoun, R. N., Rinehart, G. J., Hathaway, A. D. & Buhler, D. D. (2005). Maximizing cultural practices to minimize weed pressure and extend herbicide treatment interval in a cool-season turfgrass mixture. Int. Turfgrass Soc. Res. J. 10(Part 2), 1184-1188.
Campbell, J. & Wallace, V. (2020). Awareness, Support, and Perceived Impact of the Connecticut Pesticide Ban. HortTechnology, 30(1), 96-101. Available at: journals.ashs.org/horttech/view/journals/horttech/30/1/article-p96.xml
Christians, N. E. (1993). The use of corn gluten meal as a natural preemergence weed control in turf. Intl Turfgrass Soc. Res. J., 7, 284-290.
Christians, N. E. (2002). Use corn gluten meal as a natural pre-emergent weed control. TurfGrass Trends, 11(1), T14-T16.
Dest, W. M., & Ebdon, J. S. (2011). Study: Natural turf use levels. SportsTurf. 27(5), 8, 10-11.
Elford, E. M. A., Tardif, F. J., Robinson, D. E. & Lyons, E. M. (2008). Effect of perennial ryegrass overseeding on weed suppression and sward composition. Weed Technol., 22, 231-239.
Grande, J. & Shortell, R. (2015). Improving turfgrass establishment with multiple-depth seeding. Golf Course Management. 84-88.
Grewal, P. S., Power, K. T., Grewal, S. K., Suggars, A. & Hauptricht, S. (2004). Enhanced consistency in biocontrol of white grubs (Coleoptera: Scarabaeidae) with new strains of entomopathogenic nematodes. Biological Control, 30, 73 - 82.
Hadley, C. H. & Hawley, I. M. (1934). General Information about the Japanese Beetle in the United States. United States Department of Agriculture Circular No. 332, December.
Henderson, J., Miller, N., Guillard, K., Harel, O., & Raman, B. (2009). Late fall sod installation produces equivalent or greater rooting strength of Poa pratensis than typical spring installations during the subsequent growing season. Applied Turfgrass Science, 6(1), 1-11.
Jordan, K. & Lyons, E. (2010, November). Effect of Irrigation Regime and Establishment on Turfgrass Stand Quality and Weed Infestation. U. Guelph, Guelph, Ontario. Abstract-ASA.
Kopp, K. L., & Guillard, K. (2002). Clipping management and nitrogen fertilization of turfgrass: Growth, nitrogen utilization, and quality. Crop Science, 42(4), 1225-1231.
Kurcab, R. (2018). Basics of how a vented turf blanket works. The Rocky Mountain Half-Time, Winter, 16-17. sportsfieldmanagementonline.com/2018/10/23/all-about-vented-turf-blankets/9837/
Kurcab, Ross. (2018). All about vented turf blankets. SportsTurf, 34(10), 32, 34-35. sturf.lib.msu.edu.ezproxy.lib.uconn.edu/article/2018oct.pdf#page=32
Landschoot, P. (2013). Using Composts to Improve Turfgrass Performance. PennState Extension. Retrieved from: plantscience.psu.edu/research/centers/turf/extension/factsheets/composts
Larson, J. L., Kesheimer, A. & Potter, D. A. (2014). Pollinator assemblages on dandelions and white clover in urban and suburban lawns. J Insect Conserv, 18, 863–873. doi.org/10.1007/s10841-014-9694-9
Lewis, J. D., Bremer, D. J., Keeley, S. J. & Fry, J. D. (2012). Wilt-based irrigation in Kentucky bluegrass: Effects on visual quality and irrigation amounts among cultivars. Crop Sci. 52(4), 1881-1890.
Lyons, E. (2009, August). Competitive turf: overseeding for weed management. Sportsfield Management, 14-16. sportsturfonline.com.
Maxey, G., Henderson, J. & Wallace, V. (2020). The Effect of Turfgrass Species, Cultivar and Seeding Rate on Playing Surface Quality when Overseeding Non-Irrigated, Pesticide-Free Sports Fields. ITSRJ.
McGraw, B. & Holdrege, T. (2012). Use of aerators to mechanically control white grub populations in turfgrass. New York State Turfgrass Association Final Report.
McCauley, R., Pinnix, D. & Miller, G. (2019). Fraise Mowing impacts soil physical properties of bermudagrass surfaces. Agron. Abr, 119401.
McNitt, A. S., Petrunak, D. M. & Uddin, W. X. (2004). Evaluation of spent mushroom substrate as a topdressing to established turf. Annu. Res. Rep. [Penn State], 102-110.
Miller, N. A., & Henderson, J. J. (2012). Organic management practices on athletic fields: Part 1: The effects on color, quality, cover, and weed populations. CropScience, 52(2), 890-903.
Minner, D. D., Valverde, F. J. & Pirtle, R. M. (2008). Seeding rates that maximize turf cover when sown during traffic. Acta Hortic. 783, 57-65.
Neal, J. & Senesac, A. (2018). Are there alternatives to glyphosate for weed control in landscapes? North Carolina State University Publications. content.ces.ncsu.edu/are-there-alternatives-to-glyphosate-for-weed-control-in-landscapes.
Park, B. (2014). Organic management and other systems employed in maintaining turfgrass. SportsTurf, 30(5), 10-12, 14.
Park, B., Samaranayake, H. & Murphy, J. (2017). Response of Tall Fescue and Kentucky Bluegrass Mixtures to Wear. ITSRJ, (13), 346-353.
Park, B. & Murphy, J. (2014). Management of Natural Turf Sports Fields. NJ Turfgrass Proceedings, NJAES, 225-234.
Pease, B., Thoms, A. & Christians, N. (2018). Effects of Wetting Agent Use to Reduce Turf Damage on Native Soil Athletic Fields. Farm Progress Reports, 2017(1), Article 64. DOI: https://doi.org/10.31274/farmprogressreports-180814-2044
Potter, D. A. (1998). Destructive Turfgrass Insects; Biology Diagnosis and Control. Sleeping Bear Press, Inc.
Puhalla, J., Krans, J., & Goatley, M. (1999). Sports Fields; A Manual for Design, Construction and Maintenance. Sleeping Bear Press, Inc.
Shelley, M. & Serensits, T. (2012). Is tall fescue right for your field? Sportsfield Management, 8-11. sportsturfonline.com
Sherratt, P. (2021). Tetraploid Ryegrass. Sports Field Management Online. https://sportsfieldmanagementonline.com/2021/04/27/tetraploid-ryegrass/12627/
Sitko, C. & Rossi, F. (2019). Non-Chemical Weed Control in Amenity Grasslands via Fraise Mowing. Abstract. ASA Meeting.
Siva, C. (2014). Alternative Strategies for Broadleaf Weed Management in Residential Lawns. U. Guelph., Guelph, Ontario.
Smiley, R. W., Dernoeden, P. H. & Clarke, B. B. (1993). Compendium of Turfgrass Disease: Second Edition. APS Press.
Smith-Fiola, D. & Gill, S. (2014). Iron-Based Herbicides: Alternative Materials for Weed Control in Landscapes and Lawns. University of Maryland Extension. Iron-based Herbicides | University of Maryland Extension (umd.edu)
Stier, J. C., Koeritz, E. J. & Garrison, M. (2008). Timing the establishment of Kentucky bluegrass: Perennial ryegrass mixtures for football fields. HortScience, 43(1), 240-244.
St. John, R., & DeMuro, N. (2013). Efficacy of corn gluten meal for common dandelion and smooth crabgrass control compared to nitrogen fertilizers. [Online]. Appl. Turfgrass Science, 1-8.
Tencza, B. J. & Henderson, J. J. (2011). Determining the importance of leaf compost topdressing when managing athletic fields organically. 2011 Turfgrass Res. Rep. [CT], 46-52.
University of Missouri Extension, Soil Testing and Plant Diagnostics Services. (2011). Compost Analysis. Retrieved August 2013 from: http://soilplantlab.missouri.edu/soil/compost.aspx
Vanini, J. T., & Rogers III, J. N. (2008). Mowing strategies and fertilization improves sports fields during and after 70-day re-establishment window. [Online]. Appl. Turfgrass Science, 1-10.
Wallace, V., Inguagiato, J. & Siegel-Miles, A. (2019). Biological Pest Control Products Available for Connecticut School Grounds. UConn Extension. ipm.cahnr.uconn.edu.
Wallace, V. & Siegel-Miles, A. (2018). Using Weather Station Data on Connecticut School Grounds and Athletic Fields. UConn Extension. ipm.cahnr.uconn.edu.
Authors
Jason Henderson, Ph.D.
Associate Professor,
Turfgrass and Soil Sciences
jason.henderson@uconn.edu
Victoria Wallace
Extension Educator,
Sustainable Turf & Landscape
victoria.wallace@uconn.edu
Websites:
Home | Turfgrass Science (uconn.edu); Home | Integrated Pest Management (uconn.edu)
Acknowledgements
The authors would like to thank the following individuals for their time and expertise reviewing the second edition of this BMP document.
Karl Guillard, Ph.D. Professor, Agronomy, Department of Plant Science and Landscape Architecture, University of Connecticut
John Inguagiato, Ph.D. Associate Professor, Turfgrass Pathology, Department of Plant Science and Landscape Architecture, University of Connecticut
Steve Rackliffe, CGCS, Extension Professor Turfgrass Science, Department of Plant Science and Landscape Architecture, University of Connecticut
Alyssa Siegel-Miles, Research Technician, Sustainable Turf & Landscape, UConn Extension
© 2020 University of Connecticut. UConn is an equal opportunity employer and program provider.
Bulletin B-0200 – September 2020
All photos by V. Wallace or J. Henderson except where noted.
Cover page photos by Alyssa Siegel-Miles, Jason Henderson, Brian Tencza, and Ben Polimer.